GMP Process Chemical
GMP Manufactured ProductURACIL, GMP Grade
Product Code: URAC-4202 | Previously: UC4202
Intended For Use In Pharmaceutical GMP Processes
Uracil is important for the detoxification of many carcinogens and is also used to detoxify many drugs such as cannabinoids and opioids. Uracil can be used for drug delivery and as an intermediate to compounds used in anticancer drugs. Other derivatives are used in pesticides and antiphotosynthetic herbicides as well as in the synthesis of caffeine. Uracil is used as a coenzyme and allosteric regulator during biochemical reactions and for polysaccharide biosynthesis and transportation of sugars containing aldehydes.
Product Specifications
| ANALYSIS | SPECIFICATIONS | ||
|---|---|---|---|
|
White to Slightly Yellow Powder | ||
|
97.0-102.0% | ||
|
Passes Test | ||
|
Passes Test | ||
|
Passes Test | ||
|
100 CFU/g max. 1000 CFU/g max. |
||
|
Assay (Manual Titration) |
≥ 98.0% |
COAs and Tech Docs
- Printable Spec Sheet
- Product Inquiry
- C of As
- SDS
- Regulatory Packet
- Process Flow Diagram
- GMP Statement
- Animal Origin Statement
- BSE/TSE
- Allergen
- Mycotoxin/Aflatoxin
- GMO Statement
- Melamine
- Residual Solvents
- Gluten Free
- Ingredient Declaration
- Prop 65
- Supply Chain
- Imidazole
- TUPP Report - Stroudsburg
- Formaldehyde
- Slip Agent Statement
- Genotoxic Impurities
- Phthalate, Bisphenol, Dioxin
- Catalyst
- Test Methods
- IRGAFOS
- Degradation and Impurity
- Stability Report
- Process Description
- Nitrosamine Risk Assessment
- Elemental Impurity Assessment
- Label Copy
- Certificate of Origin
- TUPP Bangor
- Kosher / Halal Statement

URAC-4202 | UC4202
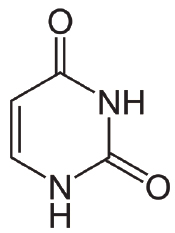
CAS #: 66-22-8
Formula: C4H4N2O2
F.W.: 112.09 g/mol
General Product Description:
- The manufacturing of Uracil URAC-4202 is performed at BioSpectra’s Bangor, PA facility utilizing multi-use equipment. Equipment used in the manufacturing of URAC-4202 is cleaned in accordance with BioSpectra’s Process Cleaning Validation Master Plan.
- Uracil is a white to slightly yellow powder.
- Molecular Formula: C4H4N2O2
- Molecular Weight: 112.09 g/mol.
- CAS Number: 66-22-8.
- There are no known major food allergens (as defined by FDA and WHO) in the manufacture of this product.
- BioSpectra certifies that all Uracil URAC-4202 manufactured at BioSpectra and its raw materials are not derived from or come in contact with animal parts, products, and/or byproducts.
- Uracil manufactured at BioSpectra and any raw materials used in the manufacture of Uracil at BioSpectra are not subject to genetic modification.
- Synonyms: 2,4-Dihydroxypyrimidine; 2,4(1H,3H)-Pyrimidinedione;
2,4-Pyrimidinediol.
GMP Compliance:
Bio Pharma Grade Uracil URAC-4202 is suitable for use as a process chemical. It is manufactured in accordance with the IPEC-PQG Joint Good Manufacturing Practice Guide. This grade of Uracil is not suitable to be used as an Active Pharmaceutical Ingredient, Drug, Drug Product or Household Item.
Retest Date:
The recommended expiration period for Uracil is three years from the date of manufacture.
Storage and Shipping Conditions:
Ship and Store in ambient temperature.
Package Sizes:
10kg, 25kg and 50kg pails.
Additional Packaging Information
https://www.biospectra.us/technical/packaging


