GMP Process Chemical
GMP Manufactured ProductL-CYSTINE Dihydrochloride
GMP Grade
Product Code: LCYS-4250 | Previously: CY4250
Intended For Use In Pharmaceutical GMP Processes
L-Cystine is a dimer of two Cysteine residues and is a non-essential amino acid. It is synthesized from non-animal derived raw materials under appropriate controls for use in Pharm and Bio-Pharm production under a fully validated GMP manufacturing system. It is also suitable for cell culture media component for the commercial biomanufacturer of therapeutic recombinant proteins and monoclonal antibodies.
Product Specifications
| ANALYSIS | SPECIFICATIONS | ||
|---|---|---|---|
|
White to Slightly Yellow Crystalline Powder | ||
|
98.0% - 102.0% | ||
|
≤ 100 CFU/g | ||
|
22.2% - 23.5% | ||
|
<0.02 EU/mg | ||
| Heavy Metals (Pb) |
<10 ppm |
||
|
Passes Test |
||
|
0.5% max. | ||
|
Report | ||
|
0.1% max. | ||
|
-225.0° to -215.0° | ||
| Solubility | Passes Test |
COAs and Tech Docs
- Printable Spec Sheet
- Product Inquiry
- C of As
- Elemental Impurities
- Regulatory Packet
- Process Flow Diagram
- GMP Statement
- Animal Origin Statement
- BSE/TSE
- Allergen
- Mycotoxin/Aflatoxin
- GMO Statement
- Melamine
- Residual Solvents
- Gluten Free
- Ingredient Declaration
- Prop 65
- Stability Data Statement
- Supply Chain
- Imidazole
- TUPP Report - Stroudsburg
- Formaldehyde
- Slip Agent Statement
- Nitrosamine Statement
- Genotoxic Impurities
- Phthalate, Bisphenol, Dioxin
- Catalyst
- Test Methods
- IRGAFOS
- Stability Report
- Nitrosamine Risk Assessment
- Certificate of Origin
- TUPP Statement
- Label Copy - US
- Label Copy - EU
- SDS – US
- SDS – EU
- Supplier Qualification
- Approved Supplier Validation Study
- Infrared Spectroscopy (IR) Scan
- Kosher / Halal Statement
- Glycol Statement

LCYS-4250
CAS #: 30925-07-6
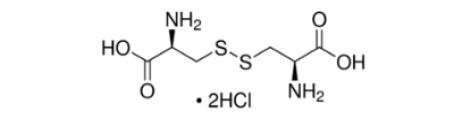
Formula:
C6H12N2O4S2·2HCl
MDL Number: MFC00070399
EC#: 250-391-9
F.W.: 313.22 g/mol
General Product Description:
- L-Cystine dihydrochloride is a White to Pale Yellow Crystalline Powder.
- Molecular Formula: C6H12N2O4S2·2HCl
- Molecular Weight: 313.22 g/mol.
- CAS Number: 30925-07-6
Physical and Chemical Properties:
Form: Solid
Color: White to Pale Yellow
Melting Point: 228-232 oC
Relative Density:1.5178 g/cm3at 20oC
Soluble in Water
GMP Compliance:
Bio Pharma Grade L-Cystine DiHydrochloride LCYS-4250 is suitable for use as a process chemical. It is GMP manufactured by the approved supplier in accordance with the approved supplier’s ISO 9001:2015 certified management system. This grade of L-Cystine DiHydrochloride is not suitable to be used as an Active Pharmaceutical Ingredient, Drug, Drug Product or Household Item.
Retest Date:
The recommended retest period for L-Cystine dihydrochloride is two years from the date of manufacture.
Storage and Shipping Conditions:
Keep away from heat. Keep away from sources of ignition. Keep container tightly closed, store in a dry and well ventilated place.
GHS Classification:
- Pictogram:


- Signal word: Warning
Hazardous Statements:
H315 - Causes skin irritation
H320 - Causes eye irritation
Package Sizes:
10kg and 25kg pails
Country of Origin:
India
Additional Packaging Information
https://www.biospectra.us/technical/packaging


