Excipient
ICH-Q7 GMP Manufactured ProductUREA, USP, GMP Excipient Grade
Product Code: UREA-3220 | Previously: UR3220
Intended For Use As An Excipient
Urea is used in biochemistry and molecular biology as a protein denaturant with low UV absorptivity. In addition to increasing solubility of hydrophobic molecules, unfolding proteins and altering their three-dimensional structures, Urea also renatures protein structures. BioSpectra manufactures repurified, GMP Urea in its FDA registered, US facility.
Product Specifications
| ANALYSIS | SPECIFICATIONS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Alcohol Insoluble Matter | 0.04% max. | |||||||||
| Appearance and Color | White / Crystals | |||||||||
| Assay | 98.0 - 102.0% | |||||||||
|
|
|||||||||
| Heavy Metals | 10 ppm max. | |||||||||
| Identification A (IR) | Passes Test | |||||||||
| Identification B | Passes Test | |||||||||
|
|
|||||||||
| Insoluble Matter |
0.010% max. |
|||||||||
| Loss on Drying |
1.0% max. |
|||||||||
| Melting Range | 132-135˚C | |||||||||
| Residue on Ignition | 0.010% max. | |||||||||
|
|
| Key Compliance Attributes of BioSpectra Grades | Bio Excipient Grade ICH-Q7 Compliant Manufactured | ||
|---|---|---|---|
|
 |
||
|
 |
||
|
 |
||
|
 |
||
|
 |
||
|
 |
||
|
 |
||
|
 |
||
|
 |
||
|
 |
||
|
 |
||
|
 |
||
|
 |
||
|
 |
||
|
 |
||
|
 |
||
|
 |
||
|
 |
||
|
 |
||
|
 |
||
|
 |
||
|
 |
||
|
 |
||
|
 |
||
|
 |
||
|
 |
||
|
 |
||
|
 |
 indicates an attribute or level of compliance which is granted or available based on the purchase of the product grade. indicates an attribute or level of compliance which is granted or available based on the purchase of the product grade. |
| Bio Excipient Grade: Intended for use as ICH-Q7 Compliant Active Pharmaceutical Ingredient |
| LBLE: LBLE applies when product specifications include requirements for Bioburden Testing (TAMC/TYMC and/or Endotoxin). LBLE stands for Low Bioburden, Low Endotoxin non-sterile products suitable for further use in parenteral manufacturing and other sterile applications. |
COAs and Tech Docs
- Printable Spec Sheet
- Product Inquiry
- Compliance
- C of As
- SDS
- Regulatory Packet
- Process Flow Diagram
- GMP Statement
- Animal Origin Statement
- BSE/TSE
- Allergen
- Mycotoxin/Aflatoxin
- GMO Statement
- Melamine
- Residual Solvents
- Gluten Free
- Ingredient Declaration
- REACH
- Prop 65
- Stability Data Statement
- Supply Chain
- Imidazole
- TUPP Report - Stroudsburg
- Formaldehyde
- Slip Agent Statement
- Genotoxic Impurities
- Phthalate, Bisphenol, Dioxin
- Catalyst
- Test Methods
- IRGAFOS
- Assay/Organic Impurities
- Stability Report
- Nitrosamine Risk Assessment
- Elemental Impurity Assessment
- Label Copy
- Certificate of Origin
- USMCA Statement
- UPLC Report

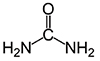
UREA-3220 | UR3220
CAS #: 57-13-6
Formula: CH4N2O
Sol. In H2O (g/L): 480 @ 20˚C
F.W.: 60.06 g/mol
pH @ 20°C: 7.2 (10% soln.)
General Product Description:
- The manufacturing of Urea UREA-3220 is performed at BioSpectra’s Stroudsburg, PA facility and is conducted in a dedicated processing area using only dedicated equipment.
- Urea is a White Crystalline product.
- Molecular Formula: CH4N2O
- Molecular Weight: 60.06 g/mol.
- CAS Number: 57-13-6.
- There are no known major food allergens (as defined by FDA and WHO) in the manufacture of this product.
- BioSpectra certifies that all Urea UREA-3220 manufactured at BioSpectra and its raw materials are not derived from or come in contact with animal parts, products, and/or byproducts.
- Urea manufactured at BioSpectra and any raw materials used in the manufacture of Urea at BioSpectra are not subject to genetic modification.
- Synonyms: Carbamide, Carbonyldiamide.
GMP Compliance:
Bio Excipient Grade Urea, UREA-3220 is suitable for use as an excipient. It is manufactured in accordance with the ICH-Q7 Good Manufacturing Practice Guide. This grade of Urea is not suitable to be used as an Active Pharmaceutical Ingredient, Drug Product or Household Item.
Retest Date:
The recommended retest period for Urea is two years from the date of manufacture.
Storage and Shipping Conditions:
- Ship and Store between 15˚ and 30˚C.
- Store in clean and dry area.
- Store in the original container.
Package Sizes:
10kg, 25kg and 50kg pails.
Additional Packaging Information
https://www.biospectra.us/technical/packaging

