GMP Buffer
GMP Manufactured Process ChemicalMOPS, Free Acid, GMP Grade
Product Code: MOPS-4220 | Previously: MP4220
Intended For Use In Pharmaceutical GMP Processes
MOPS is a zwitterionic buffer used as a running buffer for denaturing gel electrophoresis and as a buffering agent in many biological and biochemical applications. MOPS interferes with the Folin protein assay and partially decomposes when autoclaved in the presence of glucose. MOPS can be used as a Good’s buffer because it has low UV absorptivity, minimal reactivity, stable pH and is soluble in water.
Product Specifications
| ANALYSIS | SPECIFICATIONS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
|
White / Crystals | |||||||||
|
99.5% min. | |||||||||
|
0.005% max. | |||||||||
|
None Detected None Detected None Detected |
|||||||||
|
Passes Test | |||||||||
|
0.1% max. | |||||||||
|
1.0% max. | |||||||||
|
3.0-4.5 | |||||||||
|
2.5-4.5 | |||||||||
|
7.0-7.5 |
|||||||||
|
0.1% max. | |||||||||
|
Passes Test | |||||||||
|
0.005% max. | |||||||||
|
5 ppm max. 5 ppm max. 5 ppm max. 5 ppm max. |
COAs and Tech Docs
- Printable Spec Sheet
- Product Inquiry
- C of As
- SDS
- Regulatory Packet
- Process Flow Diagram
- GMP Statement
- Animal Origin Statement
- BSE/TSE
- Allergen
- Mycotoxin/Aflatoxin
- GMO Statement
- Melamine
- Residual Solvents
- Gluten Free
- Ingredient Declaration
- REACH
- Prop 65
- Stability Data Statement
- Supply Chain
- Imidazole
- TUPP Report - Stroudsburg
- Formaldehyde
- Slip Agent Statement
- Genotoxic Impurities
- Phthalate, Bisphenol, Dioxin
- Catalyst
- Test Methods
- IRGAFOS
- Nitrosamine Risk Assessment
- Elemental Impurity Assessment
- Label Copy
- Certificate of Origin
- Product Line Regulatory Packet
- Elemental Impurity Assessment - Suite 1

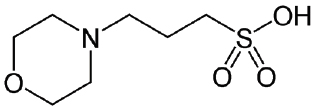
MOPS-4220
CAS #: 1132-61-2
Formula: C7H15NO4S
Sol. In H2O (g/L): 980
F.W.: 209.26 g/mol
pH @ 20°C: 3.0 - 4.5
Useful pH: 6.5 - 7.9
pKa @ 20˚C: 7.2
General Product Description:
- The manufacturing of Bio Pharma Grade MOPS MOPS-4220 is performed at BioSpectra’s Stroudsburg, PA facility and
is conducted in a dedicated processing area using only dedicated equipment. - MOPS is a White Crystalline product.
- Molecular Formula: C7H15NO4S
- Molecular Weight: 209.26 g/mol.
- CAS Number: 1132-61-2.
- There are no known major food allergens (as defined by FDA and WHO) in the manufacture of this product.
- BioSpectra certifies that all MOPS MOPS-4220 manufactured at BioSpectra and its raw materials are not derived from
or come in contact with animal parts, products, and/or byproducts. - MOPS manufactured at BioSpectra and any raw materials used in the manufacture of MOPS at BioSpectra are not subject to genetic modification.
- Synonyms: 3-(N-Morpholino)propanesulfonic acid, 4-Morpholinepropanesulfonic acid.
GMP Compliance:
Bio Pharma Grade MOPS, Free Acid MOPS-4220 is suitable for use as a process chemical. It is manufactured in accordance with the IPEC-PQG Joint Good Manufacturing Practice Guide. This grade of MOPS, Free Acid is not suitable to be used as an Active Pharmaceutical Ingredient, Drug, Drug Product or Household Item.
Retest Date:
The recommended retest period for MOPS is two years from the date of manufacture.
Storage and Shipping Conditions:
Ship and Store in ambient temperature.
Package Sizes:
10kg, 25kg and 50kg pails.
Additional Packaging Information
https://www.biospectra.us/technical/packaging


