GMP Solution
ICH-Q7 GMP Manufactured ProductUrea 6M, GMP Excipient Grade
Made With USP/EP Grade Urea And Water For Injection
Product Code: UREA-3120
Intended For Use As An Excipient Grade Solution
Urea is used as a protein denaturant with low UV absorptivity. In addition to increasing solubility of hydrophobic molecules, unfolding proteins and altering their three-dimensional structures, Urea also renatures protein structures. BioSpectra manufactures repurified, GMP Urea. This multi-compendial certified Urea crystal is combined with WFI grade water to manufacture the finished 6M Urea Solution.
Product Specifications
|
ANALYSIS |
SPECIFICATIONS |
||
|---|---|---|---|
| Appearance | Colorless Liquid | ||
|
Conforms to Standard | ||
|
5.8 – 6.2 M | ||
|
7 - 10 | ||
|
≤ 5 ppm ≤ 5 ppm ≤ 5 ppm ≤ 5 ppm |
MANUFACTURING STATEMENT: Manufactured using Urea raw material purified (in process) to Meet USP/EP compendial specifications.
COAs and Tech Docs
UREA-2220 | UR2220
CAS #: 57-13-6
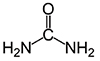
Formula:
CH4N2O
360 grams per Liter Urea
pH of a 6M soln., 25°C = 7-10
General Product Description:
The manufacturing of Bio Excipient Grade Urea 6M soln.,
UREA-3120 is performed at BioSpectra’s Bangor, PA facility and is conducted in a multi-purpose processing area using multi-purpose equipment.
- Urea 6M solution is a colorless liquid.
- Molecular Formula: CH4N2O
- Molecular Weight: 60.06 g/mol.
- 6M solution: 360 g/liter
- pH: 7-10
- CAS Number: 57-13-6.
- There are no known major food allergens (as defined by FDA and WHO) in the manufacture of this product.
- BioSpectra certifies that all Urea 6M solution, UREA-3120 manufactured at BioSpectra and its raw materials are not derived from or come in contact with animal parts, products, and/or byproducts.
- Urea 6M solution manufactured at BioSpectra and any raw materials used in the manufacture of Urea at BioSpectra are not subject to genetic modification.
- Manufactured with WFI water.
- Synonyms: Carbamide Solution, Carbonyl Diamide Solution
GMP Compliance:
Bio Excipient Grade Urea 6M solution, UREA-3120 is suitable for use as an excipient. It is manufactured in accordance with the ICH-Q7 Good Manufacturing Practice Guide. This grade of Urea 6M solution is not suitable to be used as an Active Pharmaceutical Ingredient, Drug Product or Household Item.
Retest Date:
Store in a tightly closed container. Store in dry, well-ventilated area with temperature between 15-30° C. Store away from incompatible substances.
Storage and Shipping Conditions:
Store in a tightly closed container. Store in dry, well-ventilated area with temperature between 15-30° C. Store away from incompatible substances.
Package Sizes:
200 Liter drums, 1,000 - 1,200 L totes.
Additional Packaging Information
https://www.biospectra.us/technical/packaging


