Excipient
ICH-Q7 GMP Manufactured ProductGUANIDINE HCl
NF Grade, LBLE, GMP Excipient Grade
Low Bioburden, Low Endotoxin, GMP Manufactured
Product Code: GHCL-3253
Intended For Critical Biopharma Applications
Guanidine Hydrochloride is a strong protein denaturant that functions as a chaotropic agent. As a denaturant, it acts to unfold proteins and turn them into their original polypeptide chains. As a chaotropic agent, it breaks down the structure of proteins. Guanidine Hydrochloride is commonly used inthe purification of RNA by dissociating the RNA into its nucleic acids and protein forms. At higher concentrations, Guanidine Hydrochloride decreases enzyme activity. It is also used to increase the solubility of hydrophobic molecules.
Product Specifications
| ANALYSIS | SPECIFICATIONS | ||
|---|---|---|---|
|
Acidity |
≤ 0.01% |
||
|
Appearance and Color |
White / Crystals |
||
| Appearance of solution (6 mol/l; water) | Clear and Colorless | ||
| Assay (Dried Basis) | 99.5% – 101.0% | ||
| Bacterial Endotoxins | ≤ 2.5 IU/g | ||
| Chloride (Cl) (Argentometric) | 36.5 – 37.5% | ||
| Chloride and Sulfate, Sulfate | ≤0.005% | ||
|
None Detected |
||
|
≤ 10 ppm | ||
|
Passes Test |
||
|
≤0.10 a.u. ≤0.03 a.u. ≤0.03 a.u. ≤0.20 a.u. ≤0.20 a.u. |
||
|
Meets the Requirements of test A |
||
|
≤0.005% |
||
|
≤ 0.5% |
||
|
≤ 0.01% |
||
|
184°C – 188°C |
||
|
4.5 – 6.0 |
||
|
5.0 – 6.5 |
||
|
Residue on Ignition |
≤ 0.05% | ||
| Solubility (6M) | Passes Test | ||
| Sulfated Ash (600 °C) | ≤ 0.05% | ||
| TAMC |
≤ 100 CFU/g |
||
| TYMC | ≤ 10 CFU/g | ||
| Bile-tolerant gram-negative bacteria (absent in 1 g) |
Passes Test |
||
| Candida albicans (absent in 1 g) |
Passes Test |
||
| Escherichia coli (absent in 1 g) |
Passes Test |
||
| Pseudomonas aeruginosa (absent in 1 g) |
Passes Test |
||
| Salmonella (absent in 10 g) |
Passes Test |
||
| Staphylococcus aureus (absent in 1 g) | Passes Test | ||
|
≤ 5 ppm |
||
| Water (According to Karl Fischer) | ≤ 0.5% | ||
| Water Insoluble |
≤ 0.05 % |
NOTES:
Residual solvents (ICH (Q3C)) excluded by manufacturing process.
Elemental impurity specifications have been set considering ICH Q3D (Guideline for Elemental Impurities). Class 1-3 elements are not likely to be present above the ICH Q3D option 1 limit, unless specified and indicated (*).
RESIDUAL SOLVENTS:
Based on the manufacturing process and the controlled handling, storage and analysis of this product, this product complies with the requirements and specifications listed in the current USP method <467> Tables 1, 2, 3, or 4.
COAs and Tech Docs
- Printable Spec Sheet
- Product Inquiry
- C of As
- SDS
- Elemental Impurities
- Regulatory Packet
- Process Flow Diagram
- GMP Statement
- Animal Origin Statement
- BSE/TSE
- Allergen
- Mycotoxin/Aflatoxin
- GMO Statement
- Melamine
- Residual Solvents
- Gluten Free
- Ingredient Declaration
- REACH
- Prop 65
- Stability Data Statement
- Supply Chain
- TUPP Report - Stroudsburg
- Formaldehyde
- Slip Agent Statement
- Nitrosamine Statement
- Genotoxic Impurities
- Phthalate, Bisphenol, Dioxin
- Catalyst
- Test Methods
- IRGAFOS
- Stability Report
- Label Copy
- Certificate of Origin
- Kosher / Halal Statement

GHCL-3250
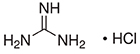
CAS #: 50-01-1
Formula: CH5N3 • HCl
Sol. In H2O (g/L): 573
F.W.: 95.53 g/mol
pH @ 20°C: 4.5 - 6.0
General Product Description:
- The manufacturing of Guanidine Hydrochloride GHCL-3253 is performed at BioSpectra’s Stroudsburg, PA facility and is conducted in a dedicated processing area using only dedicated equipment.
- Guanidine HCl is a White Crystalline product.
- Molecular Formula: CH5N3 • HCl
- Molecular Weight: 95.53 g/mol.
- CAS Number: 50-01-1.
- There are no known major food allergens (as defined by FDA and WHO) in the manufacture of this product.
- BioSpectra certifies that all Guanidine Hydrochloride GHCL-3253 manufactured at BioSpectra and its raw materials are not derived from or come in contact with animal parts, products, and/or byproducts.
- Guanidine Hydrochloride manufactured at BioSpectra and any raw materials used in the manufacture of Guanidine Hydrochloride at BioSpectra are not subject to genetic modification.
- Synonyms: Guanidine Monohydrochloride, Guanidinium Chloride, Guanidinium Hydrochloride.
GMP Compliance:
Bio Excipient Grade Guanidine Hydrochloride, GHCL- 3253 is suitable for use as an excipient. It is manufactured in accordance with the ICH-Q7 Good Manufacturing Practice Guide. This grade of Guanidine Hydrochloride is not suitable to be used as an Active Pharmaceutical Ingredient, Drug Product or Household Item.
Retest Date:
The recommended retest period for Guanidine Hydrochloride is two years from the date of manufacture.
Storage and Shipping Conditions:
Ship and store in ambient temperature. Store in a clean and dry area. Store in the original container.
Package Sizes:
10kg, 25 kg and 50 kg pails.
Additional Packaging Information
https://www.biospectra.us/technical/packaging


