GMP Solution
GMP Manufactured Process Chemical6M HCl in IPA, GMP Grade
Product Code: IHCL-4101 | Previously: IH4101
Intended For Use In Pharmaceutical GMP Processes
BioSpectra’s product “6M HCl in IPA” is a fully validated GMP compounded solution. 6M HCl in IPA is used in the manufacture of pharmaceuticals to obtain a hydrochloride salt.
Product Specifications
|
ANALYSIS |
SPECIFICATIONS |
||
|---|---|---|---|
|
Clear, colorless to slightly yellowish fuming liquid | ||
|
≥ 5.9N | ||
|
Passes Test |
COAs and Tech Docs
- Printable Spec Sheet
- Product Inquiry
- C of As
- SDS
- Elemental Impurities
- Regulatory Packet
- Process Flow Diagram
- GMP Statement
- Animal Origin Statement
- BSE/TSE
- Allergen
- Mycotoxin/Aflatoxin
- Melamine
- Residual Solvents
- Gluten Free
- Ingredient Declaration
- Prop 65
- Supply Chain
- Imidazole
- TUPP Report - Stroudsburg
- Formaldehyde
- Slip Agent Statement
- Genotoxic Impurities
- Phthalate, Bisphenol, Dioxin
- Catalyst
- Test Methods
- IRGAFOS
- Degradation and Impurity
- Stability Report
- Nitrosamine Risk Assessment
- Label Copy
- Certificate of Origin
- External validation Report 6N HCl in IPA - Formosa Gas

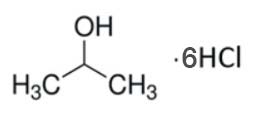
IHCL-4101
CAS #: 7647-01-0 / 67-63-0
Formula:
6M HCl / 1 LC3H8O
Solubility: Miscible with water
pH @ 20°C: <2
Density: 0.909
General Product Description:
The manufacturing of Bio Pharma Grade 6M HCl in IPA, IHCL-4101 is performed at BioSpectra’s Bangor, PA facility and is manufactured using multi-use equipment. Equipment used in the manufacturing of IHCL-4101 is cleaned in accordance with BioSpectra’s Cleaning Validation Plan.
- Molecular Formula: 6M HCl / 1 LC3H8O
- Density: 0.909
- CAS #: 7647-01-0 / 67-63-0
- 6M HCl in IPA is a clear, colorless to slightly yellowish fuming liquid.
- There are no known major food allergens (as defined by FDA and WHO) in the manufacture of this product.
- BioSpectra certifies that all 6M HCl in IPA, IHCL-4101 manufactured at BioSpectra and its raw materials are not derived from or come in contact with animal parts, products and/or byproducts.
- 6M HCl in IPA manufactured at BioSpectra and any raw materials used in the manufacture of 6M HCl in IPA at BioSpectra are not subject to genetic modification.
- Synonyms: Hydrogen Chloride in 2-Propanol Solution, Hydrochloric acid in 2-Propanol
GMP Compliance:
Bio Pharma Grade 6M HCl in IPA IHCL-4101 is suitable for use as a process chemical. It is manufactured in accordance with the IPEC-PQG Joint Good Manufacturing Practice Guide. This grade of 6M HCl in IPA is not suitable to be used as an Active Pharmaceutical Ingredient, Drug, Drug Product or Household Item.
Retest Date:
The recommended retest period for 6M HCl in IPA solution is two years from the date of manufacture.
Storage and Shipping Conditions:
Storage temperature should be kept under 55°F away from incompatible and combustible substances. Keep containers tightly closed.
GHS Classification:
Pictograms 
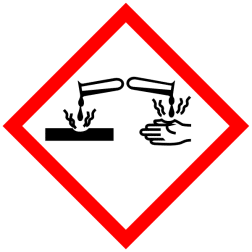
Signal Word - Danger 
Hazard Statements
H225 - Highly flammable liquid and vapor
H290 - May be corrosive to metals
H319 - Causes serious eye irritation
H336 - May cause drowsiness or dizziness
Package Sizes:
200L drums
Additional Packaging Information
https://www.biospectra.us/technical/packaging


