GMP Solution
ICH-Q7 GMP Manufactured ProductSodium Hydroxide 1.0N Soln.
GMP, LBLE, Excipient Grade
Low Chloride, Low Iron, BET tested, Made with WFI,
Sterile Filtered into Sterile Single Use Pkg.
Product Code: NAHY-3153 | Previously: NH3153
Intended For Use In Pharmaceutical GMP Processes
High Purity Sodium Hydroxide 1.0N solution is intended for use in critical pharmaceutical processes, both upstream and downstream. This product is manufactured utilizing a proprietary, fully dedicated, validated GMP system that utilizes multiple manufacturing and purification steps to achieve high purity results without the use of pellets.
Product Specifications
| ANALYSIS | SPECIFICATIONS | ||
|---|---|---|---|
|
Clear / Colorless Liquid | ||
|
≤ 5 ppm | ||
|
≤ 2.0 EU/mL | ||
|
≤ 1 ppm | ||
|
≤ 0.5 ppm | ||
| Normality | 0.9N – 1.1N | ||
|
StyLux ST 0.2 Ultra Cap HD Filter from Meissner | ||
|
Verified |
| List of C of As files |
|---|
|
NAHY-0121-0187_NAHY-3153.pdf |
|
NH3153-004-0221.pdf |
COAs and Tech Docs
- Printable Spec Sheet
- Product Inquiry
- C of As
- SDS
- Elemental Impurities
- Process Flow Diagram
- GMP Statement
- Animal Origin Statement
- BSE/TSE
- Allergen
- Mycotoxin/Aflatoxin
- GMO Statement
- Melamine
- Residual Solvents
- Gluten Free
- Ingredient Declaration
- REACH
- Prop 65
- Stability Data Statement
- Supply Chain
- Imidazole
- TUPP Report - Stroudsburg
- Formaldehyde
- Slip Agent Statement
- Genotoxic Impurities
- Phthalate, Bisphenol, Dioxin
- Catalyst
- Test Methods
- Nitrosamine Risk Assessment
- Label Copy
- Certificate of Origin

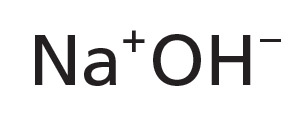
NAHY-3153
CAS #: 1310-73-2
Formula: NaOH
Density: 1.020 g/cm3 at 20°C
Storage Temp: Ambient
EC#: 215-185-5
UN: UN1824
Merck Index: 14,08627
ADR: 8,11
F.W.: 40.00 g/mol
General Product Description:
The manufacturing of Bio Excipient Grade Sodium Hydroxide NAHY-3153 is performed at BioSpectra’s Bangor, PA, US FDA registered, GMP facility and is conducted in a dedicated processing area using only dedicated equipment.
- Molecular Formula: NaOH
- Molecular Weight: 40.00 g/mol
- CAS #: 1310-73-2
- Sodium Hydroxide 1N solution is a clear, colorless liquid.
- There are no known major food allergens (as defined by FDA and WHO) in the manufacture of this product.
- BioSpectra certifies that all Sodium Hydroxide 1N solution, NAHY-3153 manufactured at BioSpectra and its raw materials are not derived from or come in contact with animal parts, products and/or byproducts.
- Sodium Hydroxide 1N solution manufactured at BioSpectra and any raw materials used in the manufacture of Sodium Hydroxide 1N solution at BioSpectra are not subject to genetic modification.
Stability and Reactivity:
Chemical Stability: Stable.
Possibility of Hazardous Reactions: Will not occur.
Incompatible Materials: Acids, organic materials, chlorinated solvents, aluminum, phosphorus, zinc, tin.
Hazardous Decomposition Products: Sodium oxides.
Physical and Chemical Properties:
Appearance: Colorless liquid.
Odor: Odorless.
Odor threshold: Not Available.
pH: ~14
Boiling range: 100°C to 140°C
Flash Point: Not flammable.
Density: 1.020 g/cm3 at 20°C
Solubility: Soluble in water.
GMP Compliance:
Bio Excipient Grade Sodium Hydroxide Solution 1N, NAHY-3153 is suitable for use as an excipient. It is manufactured in accordance with the ICH-Q7 Good Manufacturing Practice Guide. This grade of Sodium Hydroxide Solution 1N is not suitable to be used as an Active Pharmaceutical Ingredient, Drug Product or Household Item.
Retest Date:
The recommended retest period for Sodium Hydroxide 1N solution is two years from the date of manufacture.
Storage and Shipping Conditions:
Ship and store in ambient temperature, there is no impact to the product within ambient conditions of 10-40°C. Store in a clean and dry area. Store in the original container. Material will freeze at slightly lower temperatures. Keep above 16°C to prevent freezing. Warming the product will allow for full dissolution of material.
GHS Classification:
Hazard Pictogram (GHS & CLP) 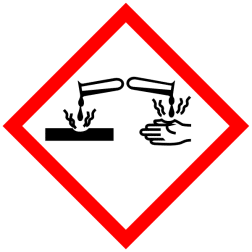
Signal Word (GHS & CLP): Danger
Hazard Statements (GHS & CLP)
H290 May be corrosive to metals
H314 Causes severe skin burns and eye damage
Package Sizes:
940L totes, 200L drums, 19L pails, 10L pails, 3.78L bottles and 6x1L case.
Additional Packaging Information
https://www.biospectra.us/technical/packaging


