Excipient
ICH-Q7 GMP Manufactured ProductTREHALOSE Dihydrate
Ultra Low Endotoxin, EP, JP, NF, LBLE, GMP
Low Bioburden, Low Endotoxin, EP, JP, NF, GMP Manufactured Excipient Grade Product
Product Code: TRED-3252 | Previously: TE3252
Intended For Use As An Excipient In Biological Drug Products
Trehalose Dihydrate is a non-reducing disaccharide used as an excipient in biotherapeutic applications. Its primary purpose is to protect the protein drug substance both in the liquid and frozen state. It provides tonicity, stabilization, cryo-protection and lyo-protection. Trehalose is superior to other sugars due to the rigidity of the alpha 1,1 bond. Trehalose is also more stable under high temperature and acidic conditions. Due to its non-reducing end, Trehalose does not react with other excipients such as amino acids or aldehydes.
Product Specifications
|
NF COMPENDIA |
||||
|---|---|---|---|---|
|
ANALYSIS |
SPECIFICATIONS |
|||
|
398.0% – 101.0% | |||
|
≤ 0.0125% |
|||
|
≤ 0.033 ≤ 0.067 |
|||
|
3≤ 0.3 EU/g |
|||
|
Conforms to Standard |
|||
|
Passes Test | |||
|
Passes Test | |||
|
Absent/g Absent/10g ≤ 50 CFU/g ≤ 20 CFU/g |
|||
|
≤ 0.005% |
|||
|
+197° to +201° |
|||
|
4.5 - 6.5 |
|||
|
≤ 0.5% ≤ 0.5% |
|||
|
≤ 0.1% |
|||
|
Passes Test | |||
|
≤ 0.0200% |
|||
|
9.0% to 11.0% |
|||
|
EP COMPENDIA |
||||
|---|---|---|---|---|
|
ANALYSIS |
SPECIFICATIONS |
|||
|
398.0% – 101.0% | |||
|
Clear, colorless |
|||
|
≤ 0.0125% |
|||
|
3≤ 0.3 EU/g |
|||
|
Conforms to Standard |
|||
|
Passes Test | |||
|
Passes Test | |||
|
≤ 0.5% ≤ 0.2% ≤ 0.2% ≤ 1.0% |
|||
|
Absent/g Absent/10g Absent Absent ≤ 50 CFU/g ≤ 20 CFU/g |
|||
|
4.5 - 6.5 |
|||
|
Passes Test |
|||
|
+197° to +201° |
|||
|
≤ 0.1% |
|||
|
≤ 0.0200% | |||
|
9.0% to 11.0% |
|||
|
JP COMPENDIA |
||||
|---|---|---|---|---|
|
ANALYSIS |
SPECIFICATIONS |
|||
|
98.0% – 101.0% | |||
|
≤ 0.018% |
|||
|
Passes Test |
|||
|
≤ 5 ppm |
|||
|
Passes Test |
|||
|
Passes Test | |||
|
Conforms to Standard |
|||
|
≤ 0.005% |
|||
|
+197° to +201° |
|||
|
4.5 – 6.5 |
|||
|
≤ 0.1% |
|||
|
≤ 0.5% ≤ 0.5% |
|||
|
≤ 0.0200% |
|||
|
9.0% to 11.0% |
|||
|
NON-COMPENDIAL ANALYSES |
|||
|---|---|---|---|
|
ANALYSIS |
SPECIFICATIONS |
||
|
White to Off-White Crystalline Powder | ||
|
≤ 200 ppm |
||
|
≤ 250 ppm |
||
|
≤ 50 ppm |
||
|
ANALYSIS |
SPECIFICATIONS |
||
|---|---|---|---|
|
≤ 50 ppb | ||
|
≤ 50 ppb |
||
|
≤ 50 ppb |
||
|
≤ 100 ppb |
||
|
Molybdenum |
≤ 100 ppb | ||
|
Copper |
≤ 100 ppb | ||
| Chromium | ≤ 100 ppb | ||
|
Iron |
≤ 100 ppb | ||
|
Aluminum |
≤ 100 ppb | ||
| Zinc | ≤ 100 ppb |
1Alternate Validated Method
2Analyses are Harmonized
3Specifications is more stringent than Compendia Monograph
COAs and Tech Docs
- Printable Spec Sheet
- Product Inquiry
- C of As
- SDS
- Regulatory Packet
- Process Flow Diagram
- GMP Statement
- Animal Origin Statement
- BSE/TSE
- Allergen
- Mycotoxin/Aflatoxin
- GMO Statement
- Melamine
- Residual Solvents
- Gluten Free
- Ingredient Declaration
- Supply Chain
- Imidazole
- TUPP Report - Stroudsburg
- Slip Agent Statement
- Genotoxic Impurities
- Phthalate, Bisphenol, Dioxin
- Catalyst
- Test Methods
- IRGAFOS
- Stability Report
- Nitrosamine Risk Assessment
- Elemental Impurity Assessment
- Label Copy
- Certificate of Origin
- External Validation Report
- Related Substances
- Impurity Profile Report
- Kosher / Halal Statement

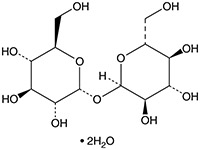
TRED-3252
CAS #: 6138-23-4
Formula: C12H22O11 • 2H2O
Sol. In H2O (g/L): 689
F.W.: 378.33 g/mol.
General Product Description:
- The Manufacturing of Trehalose, Dihydrate TRED-3252 is performed at BioSpectra’s Bangor, PA facility
- Trehalose is a White to off white Crystalline powder
- Molecular Formula: C12H22O11 · 2H2O
- Molecular Weight: 378.33 g/mol
- CAS Number: 6138-23-4
- Trehalose, Dihydrate is not manufactured with or using any of the following substances: Melamine, Latex and Glycerine.
- BioSpectra certifies that all Trehalose, Dihydrate TRED-3252 manufactured at BioSpectra and its raw materials are not derived from or come in contact with animal parts, products, and/or byproducts.
- Trehalose, Dihydrate manufactured at BioSpectra and any raw materials used in the manufacture of Trehalose, Dihydrate at BioSpectra are not subject to genetic modification.
GMP Compliance:
Bio Excipient Grade Trehalose Dihydrate TRED-3252 is suitable for use as an excipient. It is manufactured in accordance with the ICH-Q7 Good Manufacturing Practice Guide. This grade of Trehalose Dihydrate is not suitable to be used as an Active Pharmaceutical Ingredient, Drug Product or Household Item.
Retest Date:
The recommended retest period for Trehalose, Dihydrate TRED-3252 is based on current available stability data in accordance with the Stability Testing Program.
Storage and Shipping Conditions:
- Ship and Store in ambient conditions.
- Store in a clean, dry and well-ventilated area.
- Store in the original container.
Package Sizes:
10kg and 25kg pails.
Additional Packaging Information
https://www.biospectra.us/technical/packaging


