Excipient
ICH-Q7 GMP Manufactured ProductD-GALACTOSE
LBLE, EP, NF, GMP, Plant Derived
Low Bioburden, Low Endotoxin, EP, NF, GMP Manufactured,
Excipient Grade Product
Product Code: GALP-3250 | Previously: GA3250
Intended for Use in Pharma Mfg. Requiring ICH-Q7,
Excipient Grade Quality & Regulatory Compliance
D-Galactose, plant derived is intended for use upstream and downstream in biological drug manufacturing processes. For this purpose BioSpectra has categorized our product as an excipient though one of the primary functions is to be used as a nutrient in mammalian cell culture media. Given the sensitivity of these cells in regard to growth, BioSpectra’s D-Galactose is manufactured to meet high purity specifications and low bioburden and endotoxin demands.
Product Specifications
|
ANALYSIS |
SPECIFICATIONS (EP) |
||
|---|---|---|---|
|
Passes Test | ||
|
White to almost white, crystalline powder | ||
|
Passes Test | ||
|
97.0% – 102.0% | ||
|
Passes Test | ||
|
Passes Test | ||
|
Passes Test | ||
|
Passes Test | ||
|
≤ 100 CFU/g | ||
|
≤ 0.1 mg/ml | ||
|
≤ 1.0% |
||
|
≤ 0.1% | ||
|
≤ 1.0% |
|
ANALYSIS |
SPECIFICATIONS (NF) |
||
|---|---|---|---|
|
Passes Test | ||
|
Passes Test | ||
| Assay | 98.0% - 102.0% | ||
|
Passes Test | ||
|
Passes Test | ||
|
Passes Test | ||
|
Passes Test | ||
| Limit of Lead | ≤ 0.5 ppm | ||
|
Absent Absent Absent Absent ≤ 1000 CFU/g ≤ 100 CFU/g |
||
|
≤ 0.6% ≤ 0.6% ≤ 0.6% ≤ 0.6% ≤ 0.6% ≤ 0.6% ≤ 0.2% ≤ 1.0% |
||
|
≤ 0.1% | ||
|
+78.0o to +81.5o | ||
|
≤ 1.0% |
|
ANALYSIS (ADDITIONAL) |
SPECIFICATIONS |
||
|---|---|---|---|
|
≤ 2.5 EU/g | ||
|
≤ 0.1% | ||
| Lead | ≤ 0.5 ppm | ||
|
≤ 500 ppm | ||
|
≤ 5000 ppm | ||
|
≤ 100 ppm | ||
|
≤ 500 ppm |
COAs and Tech Docs
- Printable Spec Sheet
- Product Inquiry
- C of As
- SDS
- Regulatory Packet
- Process Flow Diagram
- GMP Statement
- Animal Origin Statement
- BSE/TSE
- Allergen
- Mycotoxin/Aflatoxin
- Melamine
- Residual Solvents
- Gluten Free
- Prop 65
- Stability Data Statement
- Supply Chain
- Imidazole
- TUPP Report - Stroudsburg
- Formaldehyde
- Slip Agent Statement
- Nitrosamine Statement
- Phthalate, Bisphenol, Dioxin
- Catalyst
- Test Methods
- IRGAFOS
- Degradation and Impurity
- Stability Report
- Nitrosamine Risk Assessment
- Elemental Impurity Assessment
- Label Copy
- Certificate of Origin
- Storage Statement
- Stability Indicating Report
- Stability Indicating Protocol
- Residual Solvents Test Methods
- Degradation and Impurity Suite E06
- Elemental Impurity Suite E06
- 2021 Validation Lots Real Time Stability Report

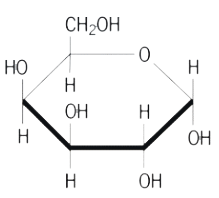
GALP-3250
CAS #: 59-23-4
Formula: C6H12O6
Sol. In H2O (g/L): 600
F.W.: 180.16 g/mol
pKa @ 25˚C: 12.4
General Product Description:
- The Manufacturing of D-Galactose, Plant Derived GALP-3250 is performed at BioSpectra’s Bangor, PA facility utilizing multiuse equipment.
- D-Galactose, Plant Derived is a White to almost white crystalline powder
- Molecular Formula: C6H12O6
- Molecular Weight: 180.16 g/mol
- CAS Number: 59-23-4
- There are no known major food allergens (as defined by FDA and WHO) in the manufacture of this product.
- BioSpectra certifies that all D-Galactose, Plant Derived GALP-3250 manufactured at BioSpectra, and its raw materials, are not derived from or come in contact with animal parts, products, and/or byproducts.
- D-Galactose, Plant Derived manufactured at BioSpectra and any raw materials used in the manufacture of D-Galactose, Plant Derived at BioSpectra are not subject to genetic modification.
- Synonyms: D-Galactopyranose
GMP Compliance:
Bio Excipient Grade D-Galactose, Plant Derived GALP-3250 is manufactured in accordance with cGMP guidelines and is suitable to be used only as the following: ICH-Q7 Compliant cGMP Manufactured non-Sterile Excipient for use in further Manufacturing. This Grade of D-Galactose, Plant Derived is not suitable to be used as a Sterile or Injectable Excipient, Active Pharmaceutical Ingredient, Drug Product or Household Item.
Retest Date:
The recommended retest period for D-Galactose, Plant Derived is two years from the date of manufacture.
Storage and Shipping Conditions:
Ship and store in ambient temperature. Keep container tightly closed in a dry and well-ventilated place.
Package Sizes:
10kg & 25kg pails.
Additional Packaging Information
https://www.biospectra.us/technical/packaging


