Reagent/Compendial
ICH-Q7 GMP Manufactured ProductTROMETHAMINE (TRIS)
99.9% High Purity, USP, Reagent GMP Grade
Low Bioburden, Low Endotoxin, Multicompendial, GMP Manufactured,
Reagent Grade Product
Product Code: TRIS-3257 | Previously: TR3257
Intended For Use As A High Purity Reagent Grade
For Critical Analytical, Diagnostic and Pharmaceutical Applications, and downstream manufacture of drug products where a high purity, high assay, GMP Tromethamine is required. Tris is a considered a “Good’s” buffer because it has low UV absorptivity, minimum reactivity, stable pH and is soluble in water.
Product Specifications
|
ANALYSIS |
SPECIFICATIONS |
|||
|---|---|---|---|---|
|
0.06 a.u. max. 0.06 a.u. max. 0.01 a.u. max. |
|||
|
0.03 a.u. max. 0.02 a.u. max. 0.004 a.u. max. |
|||
|
0.2 a.u. max. | |||
|
20 APHA max. | |||
|
99.9% min | |||
|
≤ 20 ppm | |||
|
≤ 2.5 EU/g | |||
|
None None None |
|||
|
1 ppm max | |||
|
0.005% max. | |||
|
1.0% max. | |||
|
0.3% max. | |||
|
168 - 172°C | |||
|
≤ 100 CFU/g ≤ 100 CFU/g |
|||
|
10.0 – 11.5 | |||
|
0.1% max. | |||
|
0.05% max | |||
|
≤ 1.6 ppm ≤ 1 ppm ≤ 1 ppm ≤ 1 ppm ≤ 1 ppm ≤ 5 ppm ≤ 1 ppm ≤ 1 ppm |
Residual Solvents: Based on the manufacturing process and the controlled handling, storage and analysis of this product, this product complies with the requirements and specifications listed in the current USP method <467> Tables 1, 2, 3, or 4.
| List of C of As files |
|---|
| No files available right now. |
COAs and Tech Docs
- Printable Spec Sheet
- Product Inquiry
- C of As
- SDS
- Regulatory Packet
- Process Flow Diagram
- GMP Statement
- Animal Origin Statement
- BSE/TSE
- Allergen
- Mycotoxin/Aflatoxin
- GMO Statement
- Melamine
- Residual Solvents
- Gluten Free
- Ingredient Declaration
- NAFTA Statement
- REACH
- Prop 65
- Supply Chain
- Imidazole
- TUPP Report - Stroudsburg
- Formaldehyde
- Slip Agent Statement
- Genotoxic Impurities
- Phthalate, Bisphenol, Dioxin
- Test Methods
- IRGAFOS
- Stability Report
- Nitrosamine Risk Assessment
- Elemental Impurity Assessment
- Label Copy
- Certificate of Origin
- USMCA Statement
- Latex Statement
- Unspecified Degradation Products
- Elemental Impurity Assessment: Tris Continuous

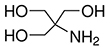
TRIS-3257 | TR3257
CAS #: 77-86-1
Formula: C4H11NO3
Sol. In H2O @ 25°C (g/L): 550
F.W.: 121.14 g/mol
pH @ 20°C 5% aq.: 10.0 - 11.5
Useful pH: 7.0 - 9.0
pKa @ 20˚C: 8.3
General Product Description:
- The manufacturing of Tromethamine TRIS-3257 is performed at BioSpectra’s Stroudsburg, PA facility and is conducted in a dedicated processing area using only dedicated equipment.
- Tromethamine is a White Crystalline product
- Molecular Formula: C4H11NO3 | Molecular Weight: 121.14 g/mol. | CAS Number: 77-86-1
- There are no known major food allergens (as defined by FDA and WHO) in the manufacture of this product.
- BioSpectra certifies that all Tromethamine TRIS-3257 manufactured at BioSpectra and its raw materials are not derived from or come in contact with animal parts, products and/ or byproducts. Tromethamine manufactured at BioSpectra and any raw materials used in the manufacture of Tromethamine at BioSpectra are not subject to genetic modification.
- Synonyms: Tris, Tromethamine, Tris(hydroxymethyl) aminomethane
Product Statements:
Plant or Animal Origin Statement: BioSpectra certifies that Tris, Product Code TRIS-3257, manufactured by BioSpectra is of Synthetic origin. BioSpectra certifies that Tris, Product Code TRIS-3257, is not derived from Plant or Animal Origin.
Metal Residue Statement: BioSpectra certifies that Tris, Product Code TRIS-3257, manufactured by BioSpectra is manufactured without the use of metal catalysts and metal reagents. There are no known risks to metal residues in this product.
Melamine Statement: Based on the manufacturing process and the controlled handling, storage, and analysis of Tris, Product Code TRIS-3257, BioSpectra certifies that there is low risk for melamine contamination. Additionally, when tested for melamine, this product reports less than 0.15 ppm.
Residual Solvents Statement: Based on the manufacturing process and the controlled handling, storage and analysis of this product, this product complies with the requirements and specifications listed in the current USP method <467> Tables 1, 2, 3, or 4.
GMP Compliance:
Bio Excipient Grade Tromethamine Multicompendial TRIS-3257 is suitable for use as an excipient. It is manufactured in accordance with the ICH-Q7 Good Manufacturing Practice Guide. This grade of Tromethamine Multicompendial is not suitable to be used as an Active Pharmaceutical Ingredient, Drug Product or Household Item.
Expiration:
The recommended expiration period for Tromethamine is three years from the date of manufacture.
Storage and Shipping Conditions:
Store in a cool, dry place. Store in a cool, dry, well- ventilated area away from incompatible substances. Keep away from acids. Keep containers tightly closed.
Package Sizes:
10 kg and 25 kg pails and 50 kg drums
Additional Packaging Information
https://www.biospectra.us/technical/packaging


