Buffer / Excipient
ICH-Q7 GMP Manufactured ProductTRIS Hydrochloride (HCl) Buffer
LBLE, LEI, Low S.A. (EP)
Low Bioburden, Low Endotoxin, GMP Manufactured
Product Code: THCL-3259 | Previously: TH3259
Intended For Use As An Excipient
Tris Hydrochloride (HCl) Buffer is a stabilizing buffer in biological applications such as electrochromatography, UV analysis and HPLC. It is used to adjust and stabilize the pH ranges for gels used in electrophoresis applications. Tris Hydrochloride (HCl) Buffer is extensively used as a biological buffer or a component of buffer solutions.
Product Specifications
|
ANALYSIS |
SPECIFICATIONS |
||
|---|---|---|---|
|
≤ 0.06 a.u. ≤ 0.06 a.u. ≤ 0.01 a.u. |
||
|
White / Crystals | ||
|
≥ 99.5% - 101% | ||
| Bioburden | ≤ 100 CFU/g | ||
| Endotoxin | ≤ 2.5 EU/g | ||
|
None None None |
||
|
2 ppm max. | ||
|
Passes Test Passes Test |
||
|
0.001% max. | ||
|
≤ 0.5% | ||
|
150–152ºC | ||
|
4.0-5.0 3.5-5.0 |
||
|
8.0 – 8.4 | ||
| Residue on Ignition | 0.1% max. | ||
|
Passes Test | ||
|
≤ 300 ppm | ||
|
1 ppm max. |
||
|
Water (Karl Fischer) |
≤ 0.5% |
General Product Description:
- The manufacturing of Tris Hydrochloride (HCl) Buffer THCL-3259 is performed at BioSpectra’s Stroudsburg, PA facility and is conducted in a dedicated processing area using only dedicated equipment.
- Tris Hydrochloride (HCl) Buffer is a White Crystalline product
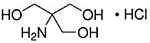
- Molecular Formula: C4H11NO3 • HCI
- Molecular Weight: 157.60 g/mol.
- CAS Number: 1185-53-1
- There are no known major food allergens (as defined by FDA and WHO) in the manufacture of this product.
- BioSpectra certifies that all Tris Hydrochloride (HCl) Buffer THCL-3259 manufactured at BioSpectra and its raw materials are not derived from or come in contact with animal parts, products and/or byproducts.
- Tris Hydrochloride (HCl) Buffer manufactured at BioSpectra and any raw materials used in the manufacture of Tris Hydrochloride at BioSpectra are not subject to genetic modification.
- Synonyms: Tris Hydrochloride (HCl) Buffer, 2-Amino-2-(Hydroxymethyl)- 1,3-Propanediol Hydrochloride; Tris (Hydroxymethyl) Aminomethane Hydrochloride
GMP Compliance:
Bio Excipient Grade Tris Hydrochloride (HCl) Buffer THCL-3259 is suitable for use as an excipient. It is manufactured in accordance with the ICH-Q7 Good Manufacturing Practice Guide. This grade of Tris Hydrochloride (HCl) Buffer is not suitable to be used as an Active Pharmaceutical Ingredient, Drug Product or Household Item.
Product Statements:
RESIDUAL SOLVENTS: Based on the manufacturing process and the controlled handling, storage and analysis of this product, this product complies with the requirements and specifications listed in the current USP method <467> Tables 1, 2, 3, or 4.
Expiration:
The recommended expiration period for Tromethamine Hydrochloride is three years from the date of manufacture..
Storage and Shipping Conditions:
Ship and store in ambient temperature. Store in a clean and dry area. Store in the original container.
Package Sizes:
10kg, 25kg and 50kg drums.
Additional Packaging Information
https://www.biospectra.us/technical/packaging
COAs and Tech Docs
- Printable Spec Sheet
- Product Inquiry
- C of As
- SDS
- Elemental Impurities
- TUPP Report - Stroudsburg
- Test Methods
- Stability Report
- Nitrosamine Report
- Elemental Impurity Assessment
- Label Copy
- Certificate of Origin
- IR Spectra
- Latex Statement
- Regulatory Packet – Stroudsburg
- Regulatory Packet – Bangor
- Allergen Statement Bangor
- Allergen Statement Stroudsburg
- Animal Origin Statement Bangor
- Animal Origin Statement Stroudsburg
- BSE / TSE Statement Bangor
- BSE / TSE Statement Stroudsburg
- Catalysts Statement Bangor
- Catalysts Statement Stroudsburg
- Certificate of Origin Statement Bangor
- Certificate of Origin Statement Stroudsburg
- Formaldehyde Statement Bangor
- Formaldehyde Statement Stroudsburg
- Genotoxic Impurity Statement Bangor
- Genotoxic Impurity Statement Stroudsburg
- Gluten Free Statement Bangor
- Gluten Free Statement Stroudsburg
- GMO Statement Bangor
- GMO Statement Stroudsburg
- GMP Statement Bangor
- GMP Statement Stroudsburg
- Imidazole Statement Bangor
- Imidazole Statement Stroudsburg
- Ingredient Declaration Bangor
- Ingredient Declaration Stroudsburg
- IRGAFOS Bangor
- IRGAFOS Stroudsburg
- Melamine Bangor
- Melamine Stroudsburg
- Mycotoxin-Aflatoxin Statement Bangor
- Mycotoxin-Aflatoxin Statement Stroudsburg
- Nitrosamine Risk Assessment Bangor
- Nitrosamine Risk Assessment Stroudsburg
- Phthalate, Bisphenol, Dioxin Bangor
- Phthalate, Bisphenol, Dioxin Stroudsburg
- Process Flow Diagram Bangor
- Process Flow Diagram Stroudsburg
- Prop 65 Bangor
- Prop 65 Stroudsburg
- REACH Bangor
- REACH Stroudsburg
- Residual Solvents Bangor
- Residual Solvents Stroudsburg
- Slip Agent Statement Bangor
- Slip Agent Statement Stroudsburg
- Supply Chain Statement Bangor
- Supply Chain Statement Stroudsburg
- Compliance Statement Bangor
- Compliance Statement Stroudsburg
- TUPP Bangor
- M11 TUPP Report Bangor
- Elemental Impurity Assessment Bangor (M11)
- Elemental Impurity Assessment: S03 Process Validation 2023
- Kosher / Halal Statement

THCL-3259 | TH3259
CAS #: 1185-53-1
Formula: C4H11NO3·HCl
Sol. In H2O (g/L): 1,010
F.W.: 157.60 g/mol
pH @ 20°C: 4.5-6.0
Useful pH: 7.0-9.0
pKa @ 20˚C: 8.2


