GMP Process Chemical
GMP Manufactured ProductDEXTRAN SULFATE 8000 Na,
LBLE, GMP Grade
Low Bioburden, Low Endotoxin, GMP Manufactured
Product Code: DXSE-4250 | Previously: DS4250
Intended for Use in Pharmaceutical GMP Processes
Dextran Sulfate 8000 Na is a polyanionic derivative of dextran, produced by the esterification of the glucose polymer (glucan polysaccharide) with chlorosulphonic acid. Dextran fractions are characterized by their average MW and MW distribution with predominantly 1-6 glycosidic bones and 5% or less of 1-3 branching. Each base dextran polymer has characteristics unique to the specific strain of bacteria from which is was derived. In addition to variables in molecular weight and branching, the level of sulfonation adds to the unique character and performance of the finished product in terms of its intended end use. Dextran Sulfate 8000 Na, 8000 MW is used in the solubilization and purification process of protein molecules intended for use in a final drug product. Dextran is neutral in pH and soluble in water. It is easily filtered and biodegradable.
Product Specifications
|
ANALYSIS |
SPECIFICATIONS |
||
|---|---|---|---|
|
Off White to light yellow powder |
||
|
≤ 0.9 OD unit |
||
|
≤ 1000 ppm |
||
|
Endotoxin |
≤ 0.012 EU/mg |
||
|
≤ 0.2% |
||
|
Glucose |
35 - 48% |
||
|
Passes Test |
||
|
Insoluble Iron |
≤ 2% |
||
| Loss on Drying | ≤ 10% | ||
| Manganese (As Reported) | ≤1ppm | ||
|
pH (1% Solution) |
5.0 to 7.5 |
||
|
Residue on Ignition |
35 - 50% |
||
|
Pyridine |
≤ 2% |
||
|
Specific Rotation [α]D20 |
+75° to 105° |
||
|
Specific Viscosity (In 1.0M NaCl at 25°C) |
0.018 – 0.032 |
||
|
Total Bioburden |
≤100CFU/g |
||
|
Sulfur |
17 - 20% |
COAs and Tech Docs
- Printable Spec Sheet
- Product Inquiry
- C of As
- SDS
- Regulatory Packet
- Process Flow Diagram
- BSE/TSE
- Residual Solvents
- Test Methods
- Long Term Stability
- Degradation and Impurity
- Nitrosamine Risk Assessment
- Validation Report
- Elemental Impurity Assessment
- Materials of Conflict Statement
- Stability Indicating Report
- Elemental Impurities Method of Analysis (ICP-MS)
- Analytical Method, Quantification of Sulfur (ICP-OES)
- Method Validation Report: Elemental Impurities
- Non-Slip Liner Compliance Statement- EU
- Test Methods – DS8 Solutions

DXSE-4250
CAS #: 9011-18-1
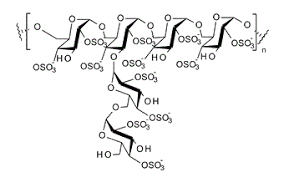
Formula:
M.W.: 8000 g/mol
pH @ 20°C: 5.0 – 7.5
General Product Description:
- The manufacturing of Dextran Sulfate 8000 Na, DXSE-4250 is performed at BioSpectra’s Bangor, PA facility utilizing multi-use equipment. Equipment used in the manufacturing of DXSE-4250 is cleaned in accordance with BioSpectra’s Process Cleaning Validation Master Plan.
- Dextran Sulfate 8000 Na is an off white to light yellow powder
- Molecular Formula: (C6H7Na3O14S3)n
- Molecular Weight: 8000 g/mol.
- CAS Number: 9011-18-1
- There are no known major food allergens (as defined by FDA and WHO) in the manufacture of this product.
- BioSpectra certifies that all Dextran Sulfate 8000 Na, DXSE-4250 manufactured at BioSpectra and its raw materials are not derived from or come in contact with animal parts, products, and/or byproducts.
- Dextran Sulfate 8000 Na manufactured at BioSpectra and any raw materials used in the manufacture of Dextran Sulfate 8000 Na at BioSpectra are not subject to genetic modification.
- Synonyms: Dextran, Hydrogen Sulfate, Sodium Salt
GMP Compliance:
Bio Pharma Grade Dextran Sulfate 8000 Na, DXSE-4250 is suitable for use as a process chemical. It is manufactured in accordance with the IPEC-PQG Joint Good Manufacturing Practice Guide. This grade of Dextran Sulfate 8000 Na is not suitable to be used as an Active Pharmaceutical Ingredient, Drug, Drug Product or Household Item.
Retest Date
The recommended expiration period for Dextran Sulfate 8000 Na is two years from the date of manufacture.
Storage and Shipping Conditions:
Store at Room Temperature
Package Sizes:
10kg, 25kg and 50kg pails.
Additional Packaging Information
https://www.biospectra.us/technical/packaging


