Excipient
ICH-Q7 GMP Manufactured ProductSynthetic Glycerin, USP, EP, JP and Ch.P.,
Parenteral Grade, GMP Excipient Grade
Product Code: GLYS-3150
Intended For Use As A Parenteral Excipient
Synthetic Parenteral Glycerin (Glycerol) is a fully synthetic, parenteral grade, suitable for use in insulin manufacturing and other critical cGMP manufacturing applications and pharmaceutical preparations.
Product Specifications
| USP COMPENDIA | |||
|---|---|---|---|
|
ANALYSIS |
SPECIFICATIONS |
||
|
99.0 to 101.0% | ||
|
≤ 10ppm | ||
|
Passes Test | ||
|
Passes Test | ||
|
Conforms with reference standard ≤ 0.025%1 ≤ 0.025%1 Passes Test |
||
|
≤ 30ppm of Cl | ||
|
≤ 0.1% ≤ 0.5%1 |
||
|
≤ 0.01% |
||
| Specific gravity (at 25.0°C) |
≥ 1.249 |
||
| Sulfate |
≤ 20ppm |
||
| Water Content |
≤ 2.0%1 |
||
1Stringent specification
| EP COMPENDIA | |||
|---|---|---|---|
|
ANALYSIS |
SPECIFICATIONS |
||
|
≤ 10ppm | ||
|
Passes Test | ||
|
Clear, colorless, or almost colorless viscous liquid | ||
|
Solution is clear, colorless | ||
|
99.0 to 101.0%1 | ||
|
≤ 10ppm | ||
| Esters | Passes Test | ||
| Halogenated compounds | ≤ 35ppm | ||
|
1.470 – 1.475 Conforms with reference standard 1.258 - 1.268 |
||
|
≤ 0.025%2 |
||
| Sugars |
Passes Test |
||
| Sulfated Ash |
≤ 0.01% |
||
| Water |
≤ 2.0% |
||
1USP Specification
2Stringent specification
|
JP COMPENDIA |
|||
|---|---|---|---|
|
ANALYSIS |
SPECIFICATIONS |
||
|
Passes Test | ||
|
Passes Test | ||
|
Passes Test | ||
|
Clear, colorless, or almost colorless viscous liquid | ||
|
≤ 0.3ppm2 | ||
|
99.0 to 101.0%1 | ||
| Calcium | Passes Test | ||
| Chloride | ≤ 10ppm | ||
|
Color |
Passes Test | ||
|
Fatty Acids or Esters |
Passes Test | ||
|
Heavy Metals |
≤ 2ppm2 | ||
|
Identification, IR |
Conforms with reference standard | ||
|
Readily carbonizable substances |
Passes Test | ||
|
≤ 0.025%2 ≤ 0.025%2 ≤ 0.1% ≤ 0.5% |
||
|
≥1.470 |
||
|
Residue on Ignition |
≤ 0.01% |
||
| Specific Gravity @20°C |
≥1.258 |
||
| Sulfate |
≤ 20ppm |
||
| Water |
≤ 2.0% |
||
1USP Specification
2Stringent specification
|
Ch.P. COMPENDIA |
|||
|---|---|---|---|
|
ANALYSIS |
SPECIFICATIONS |
||
|
Passes Test | ||
|
Passes Test | ||
|
Passes Test | ||
|
Clear, colorless, or almost colorless viscous liquid | ||
|
≤ 0.3ppm | ||
|
99.0 to 101.0%1 | ||
| Bacterial Endotoxin | ≤ 10 EU/g | ||
| Calcium | Passes Test | ||
| Chloride | ≤ 6ppm | ||
|
Color |
Passes Test | ||
|
Fatty Acids or Esters |
Passes Test | ||
|
Halogenated Compounds |
≤ 30ppm | ||
|
Heavy Metals |
≤ 2ppm | ||
|
Identification, IR |
Conforms with reference standard | ||
|
Iron |
≤ 0.5ppm | ||
|
Absent/per g ≤ 100CFU/g2 ≤ 100CFU/g |
||
|
Readily carbonizable substances |
Passes Test |
||
|
Refractive Index |
1.470 - 1.475 |
||
|
Relative Density |
1.258 – 1.268 |
||
|
≤ 0.025% |
||
|
Residue of Ignition |
≤ 2mg |
||
|
Specific gravity (at 25.0°C) |
≥ 1.249 |
||
|
Sugars |
Passes Test |
||
|
Sulfates |
≤ 20ppm |
||
|
Water |
≤ 2.0% |
||
1USP Specification
2Stringent specification
| ADDITIONAL ANALYSES | |||
|---|---|---|---|
|
ANALYSIS |
SPECIFICATIONS |
||
|
≤ 5ppm | ||
|
≤ 5ppm | ||
|
≤ 5ppm | ||
|
≤ 5ppm | ||
|
≤ 3000ppm | ||
|
≤ 100CFU/g | ||
| List of C of As files |
|---|
| No files available right now. |

COAs and Tech Docs
- Printable Spec Sheet
- Product Inquiry
- C of As
- SDS
GLYS-3150
CAS #: 56-81-5
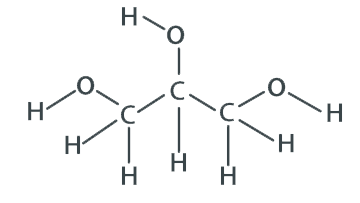
Formula: C3H8O3
F.W.: 92.1 g/mol
General Product Description:
Synthetic Glycerin is produced at our cGMP platform in India and then shipped to and fully retested and packaged at our sites in Rensselaer, NY and Bangor PA, USA.
-
Synthetic Glycerin is a Clear Liquid product.
-
Molecular Formula: C3 H8 O3
-
Molecular Weight: 92.09 g/mol
-
CAS Number: 56-81-5
-
There are no known major food allergens (as defined by FDA and WHO) in the manufacture of this product.
-
Synthetic Glycerin GLYS-3150 at BioSpectra and its raw materials are not derived from or come in contact with animal parts, products, and/or byproducts.
-
Synthetic Gycerin manufactured at our cGMP platform in India and any raw materials used in the manufacture of Synthetic Glycerin at BioSpectra are not subject to genetic modification.
-
Synthetic Gycerin is further purified at our site in Canada, Dextran Products apart of the BioSpectra Organization.
-
Synonyms: Glycerol, Glycerolum, Glicerol, Glyzerin, 1,2,3-Propantriol, Glycerinester, Pfl.
GMP Compliance:
Bio Excipient Grade Synthetic Glycerin, GLYS-3150 is suitable for use as an excipient. It is manufactured in accordance with the ICH-Q7 Good Manufacturing Practice Guide. This grade of Synthetic Glycerin is not suitable to be used as an Active Pharmaceutical Ingredient, Drug Product or Household Item.
Retest Date:
The recommended retest period for Synthetic Glycerin is two years from the date of manufacture.
Storage and Shipping Conditions:
Ship and store in ambient temperature.
Package Sizes:
6x1L, 4x 3.78L, 10L, 200L and 1000 liter
(Please contact us for more specific information about your package size and Chain of Custody.)
Country of Origin:
India
Additional Packaging Information
https://www.biospectra.us/technical/packaging


