Ultra Diagnostic / Reagent Grade
GUANIDINE THIOCYANATE
Product Code: GTHI-5221 | Previously: GT5221
Intended For Use In Commercial Manufacturing, Diagnostic And Laboratory Applications
Guanidine Thiocyanate is a chaotropic agent. As one of the strongest protein denaturants, it inactivates nucleases more than twice as fast as Guanidine Hydrochloride. Guanidine Thiocyanate is preferred for the purification of RNA because it dissociates the RNA into its nucleic acids and protein forms. Higher molarity solutions of Guanidine Thiocyanate irreversibly inactivate RNase. Guanidine Thiocyanate causes proteins to dissolve readily, thus disintegrating cellular structures.
Product Specifications
| ANALYSIS | SPECIFICATIONS | ||
|---|---|---|---|
|
White / Crystals | ||
|
≥ 99.0% | ||
|
None Detected None Detected |
||
| Heavy Metals (as Lead) | ≤ 5ppm | ||
|
Conforms to Structure | ||
|
Colorless | ||
|
Clear | ||
|
≤0.0005% ≤0.0005% ≤0.005% ≤0.5% |
COAs and Tech Docs
- Printable Spec Sheet
- Product Inquiry
- C of As
- SDS

GTHI-5221
CAS #: 593-84-0
Formula: C2H6N4S
Sol. In H2O (g/L): 1,600
F.W.: 118.16 g/mol
pH @ 20°C: 5.0 - 7.0
General Product Description:
- Guanidine Thiocyanate is a White/Colorless Crystalline or Powder product.
- Molecular Formula: C2H6N4S
- Molecular Weight: 118.16 g/mol.
- CAS Number: 593-84-0.
- Synonyms: Guanidine Isothiocyanate.
Compliance:
Material represented by this grade is suitable to be used only as the following: Quality System Manufactured Process Chemical for use in further manufacturing or as a reagent chemical for commercial manufacturing, diagnostic and laboratory research. The material represented by this grade is not suitable to be used as an Excipient, Active Pharmaceutical Ingredient, Drug, Drug Product or household item.
Retest Date:
Two years from date of manufacture, testing and packaging.
Storage and Shipping Conditions:
Ship and Store in ambient temperature.
GHS Classification:
Pictograms: 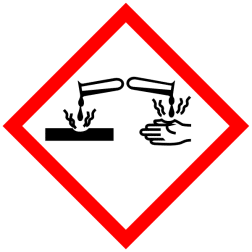

Signal Word: Danger
Hazard Statements:
H302 - Harmful if swallowed
H312 - Harmful in contact with skin
H314 - Causes severe skin burns and eye damage
H332 - Harmful if inhaled
H412 - Harmful to aquatic life with long lasting effects
Package Sizes:
10kg, 25kg and 50kg pails.
Additional Packaging Information
https://www.biospectra.us/technical/packaging

