Excipient
ICH-Q7 GMP Manufactured ProductTROMETHAMINE (TRIS)
Ch.P., GMP Excipient Grade
Product Code: TRIS-3251 | Previously: TR3251
Intended For Use As An Excipient
Tromethamine (Tris), is commonly used as a buffer and excipient in biological applications downstream in the manufacture of drug products. Tris is considered a “Good’s” buffer because it has low UV absorptivity, minimal reactivity, stable pH and is soluble in water.
Product Specifications
|
ANALYSIS |
SPECIFICATIONS |
||||||
|---|---|---|---|---|---|---|---|
|
|
||||||
|
|
||||||
|
|
||||||
|
|
||||||
|
|
||||||
|
Passes Test | ||||||
|
|
||||||
|
|
||||||
| Chloride (ChP/EP) |
|
||||||
|
Passes Test | ||||||
|
|
||||||
|
None Detected |
||||||
|
|
||||||
|
Passes Test |
||||||
|
Passes Test | ||||||
|
Passes Test |
||||||
|
Passes Test | ||||||
|
Passes Test |
||||||
|
Identification D (EP/ChP-2) |
Passes Test |
||||||
|
Insoluble Matter |
|
||||||
| Karl Fischer Water | ≤ 1.0% | ||||||
| Loss on Drying (USP/ChP/EP/JPC) | ≤ 0.3% | ||||||
| Melting Range (USP/ChP/IDB-EP/JPC) | 168 - 172°C | ||||||
| Methanol | ≤ 3000 ppm | ||||||
|
≤ 100 CFU/g |
||||||
| pH (5%) (USP/IDA-EP/ChP) | 10.0 – 11.5 | ||||||
| pH (1 in 100) (JPC) | 10.3 – 10.7 | ||||||
| Related Substances (ChP/EP) | ≤ 0.1% | ||||||
| Residue on Ignition/Sulfated Ash (USP/ChP/EP/JPC) | ≤ 0.05% | ||||||
|
≤ 1.6 ppm |
COAs and Tech Docs
- Printable Spec Sheet
- Product Inquiry
- C of As
- SDS
- Regulatory Packet
- Process Flow Diagram
- GMP Statement
- Animal Origin Statement
- BSE/TSE
- Allergen
- Mycotoxin/Aflatoxin
- GMO Statement
- Melamine
- Residual Solvents
- Gluten Free
- Ingredient Declaration
- REACH
- Prop 65
- Supply Chain
- Imidazole
- TUPP Report - Stroudsburg
- Formaldehyde
- Slip Agent Statement
- Genotoxic Impurities
- Phthalate, Bisphenol, Dioxin
- Test Methods
- IRGAFOS
- Stability Report
- Nitrosamine Report
- Nitrosamine Risk Assessment
- Elemental Impurity Assessment
- Label Copy
- Certificate of Origin
- USMCA Statement
- Latex Statement
- Unspecified Degradation Products
- Elemental Impurity Assessment: Tris Continuous
- Kosher / Halal Statement

TRIS-3251 TR3251
CAS #: 77-86-1
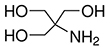
Formula: C4H11NO3
Sol. In H2O @ 25°C (g/L): 550
F.W.: 121.14 g/mol
pH @ 20°C 5% aq.: 10.0 - 11.5
Useful pH: 7.0 - 9.0
pKa @ 20˚C: 8.3
General Product Description:
- The manufacturing of Tromethamine TRIS-3251 is performed at BioSpectra’s Stroudsburg, PA facility and is conducted in a dedicated processing area using only dedicated equipment.
- Tromethamine is a White Crystalline product
- Molecular Formula: C4H11NO3
- Molecular Weight: 121.14 g/mol.
- CAS Number: 77-86-1
- There are no known major food allergens (as defined by FDA and WHO) in the manufacture of this product.
- BioSpectra certifies that all Tromethamine TRIS-3251 manufactured at BioSpectra and its raw materials are not derived from or come in contact with animal parts, products and/or byproducts. Tromethamine manufactured at BioSpectra and any raw materials used in the manufacture of Tromethamine at BioSpectra are not subject to genetic modification.
- Synonyms: Tris, Tromethamine, Tris(hydroxymethyl)aminomethane
GMP Compliance:
Bio Excipient Grade Tromethamine, Chinese Pharmacopeia TRIS-3251 is suitable for use as an excipient. It is manufactured in accordance with the ICH-Q7 Good Manufacturing Practice Guide. This grade of Tromethamine, Chinese Pharmacopeia is not suitable to be used as an Active Pharmaceutical Ingredient, Drug Product or Household Item.
Expiration:
The recommended expiration period for Tromethamine is three years from the date of manufacture.
Storage and Shipping Conditions:
Store in a cool, dry place. Store in a cool, dry, well-ventilated area away from incompatible substances. Keep away from acids. Keep containers tightly closed.
Package Sizes:
10kg, 25kg and 50kg pails.
Residual Solvents Statement:
Based on the manufacturing process and the controlled handling, storage and analysis of this product, this product complies with the requirements and specifications listed in the current USP method <467> Tables 1, 2, 3, or 4.
Additional Packaging Information
https://www.biospectra.us/technical/packaging


