Excipient
GMP Manufactured ProductL-GLUTAMINE, USP, GMP Excipient Grade
Product Code: LGLM-4250
Intended For Use As An Excipient
Glutamine is an α-amino acid that is used in the biosynthesis of proteins. This product is synthesized and purified under full GMP conditions for use in GMP Pharmaceutical Production. L-Glutamine is used as a supplement energy source for certain types of mammalian cells and is used in other biopharmaceutical manufacturing applications.
Product Specifications
| ANALYSIS | SPECIFICATIONS | ||
|---|---|---|---|
|
98.5% - 101.5% | ||
|
White crystals or crystalline powder | ||
|
≤ 0.05% | ||
| Chloride and Sulfate, Sulfate | ≤ 0.03% | ||
| Identification A (IR) |
Passes Test |
||
|
≤ 10 ppm |
||
|
≤ 0.3% | ||
|
+6.3° to +7.3° | ||
|
≤ 0.10% | ||
| Related Compounds | ≤ 0.5% |
| List of C of As files |
|---|
| No files available right now. |

COAs and Tech Docs
- Printable Spec Sheet
- Product Inquiry
- C of As
- Test Methods
- L-Glutamine Testing Methods
LGLM-4250
CAS #: 56-85-9
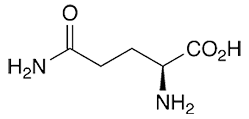
Formula:
C5H10N2O3 • 2H2O
pH (6M) @ 20°C: 4.5 - 6.0
Sol. In H2O (g/L): 36
F.W.: 146.14 g/mol
General Product Description:
- L-Glutamine is produced at our cGMP platform in India and then shipped to our Bangor, PA facility where it is tested and repackaged under cGMP conditions.
- L-Glutamine is a White Crystalline product.
- Molecular Formula: C5H10N2O3 • 2H2O
- Molecular Weight: 146.14 g/mol.
- CAS Number: 56-85-9
- There are no known major food allergens (as defined by FDA and WHO) in the manufacture of this product.
- BioSpectra certifies that all L-Glutamine, LGLM-4250 at BioSpectra and its raw materials are not derived from or come in contact with animal parts, products, and/or byproducts.
- L-Glutamine manufactured at our cGMP platform in India and any raw materials used in the manufacture of L-Glutamine at BioSpectra are not subject to genetic modification.
- Synonyms: (S)-2,5-Diamino-5-oxopentanoic acid, L- Glutamic acid 5-amide.
GMP Compliance:
Bio Pharma Grade L-Glutamine, LGLM-4250 is suitable for use as an excipient. It is manufactured in accordance with the IPEC-PQG Joint Good Manufacturing Practice Guide For Excipients. This grade of L-Glutamine is not suitable to be used as an Active Pharmaceutical Ingredient, Drug, Drug Product or Household Item.
Retest Date:
The recommended retest period for L-Glutamine is two years from the date of manufacture.
Storage and Shipping Conditions:
Ship and Store in ambient temperature.
Package Sizes:
10kg, 25kg and 50kg pails.
Country of Origin
India
This product is then repacked and retested under cGMP at our Bangor USA cGMP FDA Regulated Facility.
Additional Packaging Information
https://www.biospectra.us/technical/packaging


