Excipient
ICH-Q7 GMP Manufactured ProductDEXTRAN 70, LBLE, USP/EPGMP EXCIPIENT GRADE
Low Bioburden, Low Endotoxin, GMP Manufactured
Product Code: DX70-3250
Intended for Use as an Excipient
Dextrans are used in diverse applications ranging from Vaccines, Ophthalmic, Protein Stabilization and Bulking Agent, to Cryopreservation, Organ Preservation, Blood Cell Separation and Blood Volume Expander.
Product Specifications
|
ANALYSIS |
SPECIFICATIONS (EP) |
||
|---|---|---|---|
|
White or almost white powder |
||
|
Complies |
||
|
Complies |
||
|
+195° to +201° |
||
|
Average molecular mass |
64,000 – 76,000 Mw |
||
|
≤185,000 Mw |
||
|
Mw of 10% low fraction |
≥15,000 Mw |
||
| Residual solvent, % by GC | Complies | ||
|
Sulphated Ash |
≤0.3 % w/w |
||
|
Loss on drying (105°C, 5h) |
≤7.0 % w/w |
||
|
Total viable aerobic count (TAMC) |
≤100 CFU/g |
|
ANALYSIS |
SPECIFICATIONS (USP) |
||
|---|---|---|---|
|
Complies |
||
|
≤0.15 a.u. |
||
|
4.5 – 7.0 | ||
|
+195° to +203° |
||
|
Mn |
34,000 – 48,000 |
||
|
1.4 – 1.9 |
||
|
Viscosity, intrinsic |
24 – 29 ml/g |
||
| Nitrogen containing impurities | ≤100 ppm N | ||
|
Alcohol and related impurities |
Complies |
||
|
Sulfate |
≤0.03 % w/w |
||
|
Loss on drying (105°C, 5h) |
≤7.0 % w/w |
||
|
Bacterial endotoxins (6% sol.) |
≤0.5 EU/ml |
||
| Antigenic impurities | Complies | ||
| Safety | Complies |
| List of C of As files |
|---|
| No files available right now. |

COAs and Tech Docs
DX70-3250
CAS #: 9004-54-0
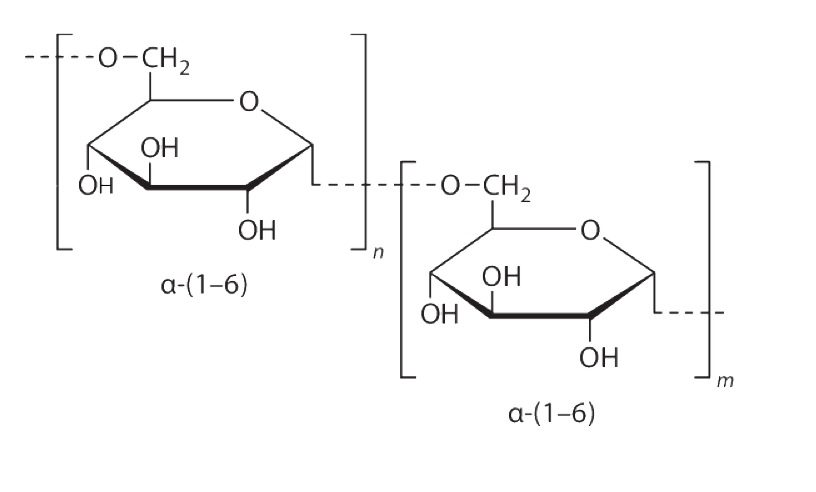
Formula:
M.W.: 63000 - 77000
pH in aqueous solution: 4.5 - 7.5%
General Product Description:
- The manufacturing of Dextran 70, DX70-3250 is performed at BioSpectra’s Rensselaer, NY facilities utilizing multi-use equipment. Equipment used in the manufacturing of DX70-3250 is cleaned in accordance with BioSpectra’s Process Cleaning Validation Master Plan.
- Dextran 70 is a White Crystalline Product.
- Molecular Formula: (C6H5O5)n
- Molecular Weight: 63000 - 77000
- CAS Number: 9004-54-0
- There are no known major food allergens (as defined by FDA and WHO) in the manufacture of this product.
- BioSpectra certifies that all Dextran 70, DX70-3250 manufactured at BioSpectra and its raw materials are not derived from or come in contact with animal parts, products, and/or byproducts.
- Dextran 70 manufactured at BioSpectra and any raw materials used in the manufacture of Dextran 70 at BioSpectra are not subject to genetic modification.
- Synonyms: Dextran
GMP Compliance:
Bio Excipient Grade Dextran 70, DX70-3250 is suitable for use as an excipient. It is manufactured in accordance with the ICH-Q7 Good Manufacturing Practice Guide. This grade of Dextran 70 is not suitable to be used as an Active Pharmaceutical Ingredient, Drug Product or Household Item.
Retest Date
The recommended expiration period for Dextran 70 is two years from the date of manufacture.
Storage and Shipping Conditions:
Ship and store in ambient temperature. Store in a clean and dry area. Store in the original container.
Package Sizes:
1kg, 5kg, 10kg, 25kg
Additional Packaging Information
https://www.biospectra.us/technical/packaging


