Excipient
ICH-Q7 GMP Manufactured ProductMES, Hydrate, LBLE, GMP Excipient Grade
Product Code: MESH-3250
Intended For Use As An Excipient
MES Hydrate is a zwitterionic buffer that is not absorbed through cell membranes and is virtually transparent in UV light. MES is a buffering agent used in many biological and biochemical applications. It is also used as a running buffer for denaturing gel electrophoresis. The characteristics of low UV absorptivity, minimal reactivity, stable pH and solubility in water allow MES Hydrate Excipient to be used as a Good’s buffer.
Product Specifications
| ANALYSIS | SPECIFICATIONS | ||
|---|---|---|---|
|
≤ 0.04 a.u. |
||
|
White Powder | ||
|
≥ 99.5% |
||
|
Passes Test | ||
|
< 50 EU/g |
||
|
Passes Test |
||
|
Identification (IR) |
Passes Test |
||
|
3.5 – 4.1 |
||
|
Clear and Colorless |
||
|
Total Aerobic Microbial Count |
≤ 100 CFU/g |
||
|
≤ 100 CFU/g |
||
|
Water (by Karl Fischer) |
5.0 – 8.9% |
||
|
≤ 5.0 ppm |
| List of C of As files |
|---|
|
MESH-0123-00003_MESH-3250.pdf |
|
MESH-0123-00002_MESH-3250.pdf |
|
MESH-0123-00001_MESH-3250.pdf |

COAs and Tech Docs
- Printable Spec Sheet
- Product Inquiry
- C of As
- SDS
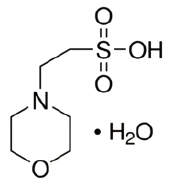
MESH-3250
CAS #: 1266615-59-1
Formula: C6H13NO4S·H2O
F.W.: 195.25 g/mol
General Product Description:
- The manufacturing of Bio Excipient Grade MES, Hydrate MESH-3250 is performed at BioSpectra’s Bangor, PA.
- MES is a White Crystalline product.
- Molecular Formula: C6H13NO4S·H2O
- Molecular Weight: 195.25 g/mol.
- CAS Number: 1266615-59.
- There are no known major food allergens (as defined by FDA and WHO) in the manufacture of this product.
- BioSpectra certifies that all MES, Hydrate MESH-3250 manufactured at BioSpectra and its raw materials are completely synthetic and are not derived from or come in contact with animal parts, products, and/or byproducts.
- Synonyms: 2-(N-Morpholino)ethanesulfonic acid, 4-Morpholineethanesulfonic acid monohydrate.
GMP Compliance:
Bio Excipient Grade MES, Hydrate MESH-3250 is suitable for use as an excipient. It is manufactured in accordance with the ICH-Q7 Good Manufacturing Practice Guide. This grade of MES, Hydrate is not suitable to be used as an Active Pharmaceutical Ingredient, Drug Product or Household Item.
Retest Date:
The recommended retest period for MES, Hydrate is two years from the date of manufacture.
Storage and Shipping Conditions:
Ship and Store in ambient temperature.
Package Sizes:
10kg, 25kg and 50kg pails.
Additional Packaging Information
https://www.biospectra.us/technical/packaging


