GMP Solution
ICH-Q7 GMP Manufactured ProductWater for Injection (WFI),
USP, EP, JP, GMP Excipient Grade
Sterile Filtered* into Bio-Compatible Sterile Single Use Pkg.
Product Code: WAFI-3150 | Previously: WI3150
Intended For Critical BioPharma Applications
Intended for use as a critical GMP Solution and Excipient for further parenteral manufacturing. *Intended for use in further parenteral manufacturing that requires terminal sterilization. Not intended for use as a sterile product.
Product Specifications
|
ANALYSIS |
SPECIFICATIONS (USP) |
||
|---|---|---|---|
|
Less than 0.25 EU/mL |
||
|
Solution Remains Faintly pink |
||
|
Meets USP/EP Requirements |
||
|
pH |
5.0 – 7.0 |
||
|
Meets USP/EP Requirements |
||
|
Meets USP/EP Requirements |
|
ANALYSIS |
SPECIFICATIONS (EP) |
||
|---|---|---|---|
|
Conforms |
||
|
Clear and Colorless Liquid |
||
|
10 ppb max. |
||
|
≤ 0.2 ppm |
||
|
Bacterial Endotoxins |
Less than 0.25 EU/mL |
||
|
Calcium and Magnesium |
A Blue Color is Produced |
||
|
Chlorides |
No Change in Appearance |
||
|
Conductivity ♦ |
Meets the Requirements |
||
|
Microbial Monitoring |
<10 CFU/100mL |
||
|
Nitrates |
0.2 ppm max. |
||
|
Solution Remains Faintly pink |
||
|
Particulate Matter |
Meets USP/EP Requirements |
||
|
pH |
5.0 – 7.0 |
||
|
Residue on Evaporation |
≤ 3.0 mg (0.003%) | ||
| Total Organic Carbon ♦ | 0.5 mg/L max. |
|
ANALYSIS |
SPECIFICATIONS (JP) |
||
|---|---|---|---|
|
Clear, colorless liquid, no odor |
||
|
Less than 0.25 EU/mL |
||
|
Not more than 2.1μS/cm-1 |
||
|
Not more than 0.50mg/L |
♦ MEETS STATED VALUE AT THE TIME OF PACKAGING
| List of C of As files |
|---|
| No files available right now. |
COAs and Tech Docs

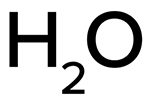
WAFI-3150
CAS #: 7732-18-5
Formula:
H2O
pH @ 25°C: 6.0 – 8.0
Melting Point: 0°C
Density: 1.00 g/cm3 @ 3.98°C
Storage Temp: 5°C to 30°C
Boiling Point: 100°C
F.W.: 18.02 g/mol
General Product Description:
The manufacturing of Bio Excipient Grade GMP WFI, WAFI-3150 is performed at BioSpectra’s Bangor, PA, US FDA registered, GMP facility and is conducted in a dedicated processing area using only dedicated equipment.
- Molecular Formula: H2O
- Molecular Weight: 18.02 g/mol
- CAS #: 7732-18-5
- GMP WFI is a clear, colorless liquid.
- There are no known major food allergens (as defined by FDA and WHO) in the manufacture of this product.
- BioSpectra certifies that all GMP WFI, WAFI-3150 manufactured at BioSpectra and its raw materials are not derived from or come in contact with animal parts, products and/or byproducts.
- GMP WFI manufactured at BioSpectra and any raw materials used in the manufacture of GMP WFI at BioSpectra are not subject to genetic modification.
GMP Compliance:
Bio Excipient Grade GMP WFI, WAFI-3150 is suitable for use as an excipient. It is manufactured in accordance with the ICH-Q7 Good Manufacturing Practice Guide. This grade of GMP WFI is not suitable to be used as an Active Pharmaceutical Ingredient, Drug Product or Household Item.
Retest Date:
The recommended retest period for GMP WFI is two years from the date of manufacture.
Storage and Shipping Conditions:
Ship and store at 5°C to 30°C
Package Sizes:
Sterile, Single use 1000L totes, 200L drums, 4L and 1L bottles.
Additional Packaging Information
https://www.biospectra.us/technical/packaging


